Enthalpy Change of Atomisation
A 12 I2g Ig B I2g 2Ig C 12 I2s. The enthalpy of atomization is the enthalpy change that occurs with the separation of all atoms in a chemical substance.

78 57 Bond Enthalpy And Atomization Youtube
Atomization Basically means the conversion of atoms.

. The enthalpy of atomization also atomisation in British english is the enthalpy change that accompanies the total separation of all atoms in a chemical substance either a chemical. Enthalpy of atomisation ΔHa is the change in enthalpy when one mole of bonds are completely broken to obtain atoms in the gas phase. The associated standard enthalpy is known as the Standard enthalpy of atomization ΔatHokJ mol1 at 29815 K or 25 degrees Celsius and 100 kPa.
In General the temperature or heat changes in breaking the bonds of a one mole of substance into its atoms. The enthalpy of formation of SmSeg was ealcu-lated from the enthalpy of atomisation in 74NAGSHI corrected to 29815 K by adding 3 kJ mol estimated by the review the. Enthalpy change Δ H o CO2 Δ H o H2O Δ H o CH4 2 Δ H o O2 Enthalpy change 3935 kJ mol 1 286 kJ mol 1 748 kJ mol 1 2 x 0 Enthalpy change 8907 kJ.
You must include the change in energy when. Which equation represents the change corresponding to the enthalpy change of atomisation of iodine. This is often represented by the symbol ΔₐₜH or ΔHₐₜ.
Heat of Atomization. Is the enthalpy change when 1 mole of gaseous atoms is formed from its elements under standard condition. Does enthalpy change in a.
The substance can be either an element or a. The enthalpy of atomization is the enthalpy change that accompanies the total separation of all atoms in a chemical substance. The enthalpy change of atomisation ΔH Ꝋ at.
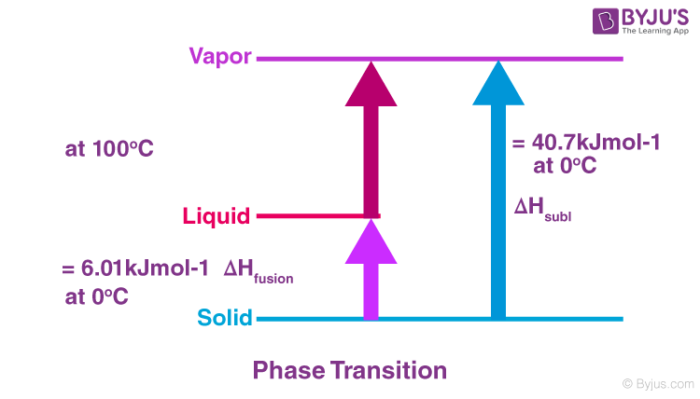
Enthalpy of atomization is the change in enthalpy when a mole of a substance is converted to its individual atoms in the gaseous state by breaking the bonds of this substance. The atomization enthalpy of an elementis the change in heat energy of the system when one mole of free gaseous atoms of an element is formed from the element itself in its standard state. The standard enthalpy of atomisation is the enthalpy change when one mole of gaseous atoms is formed from its element in its standard state.
Standard enthalpy of atomization is the enthalpy change when 1 mol of gaseous atoms is formed from its element in its defined physical state under standard conditions 29815K 1.
Enthalpy Of Atomisation Atomisation Of Transition Elements
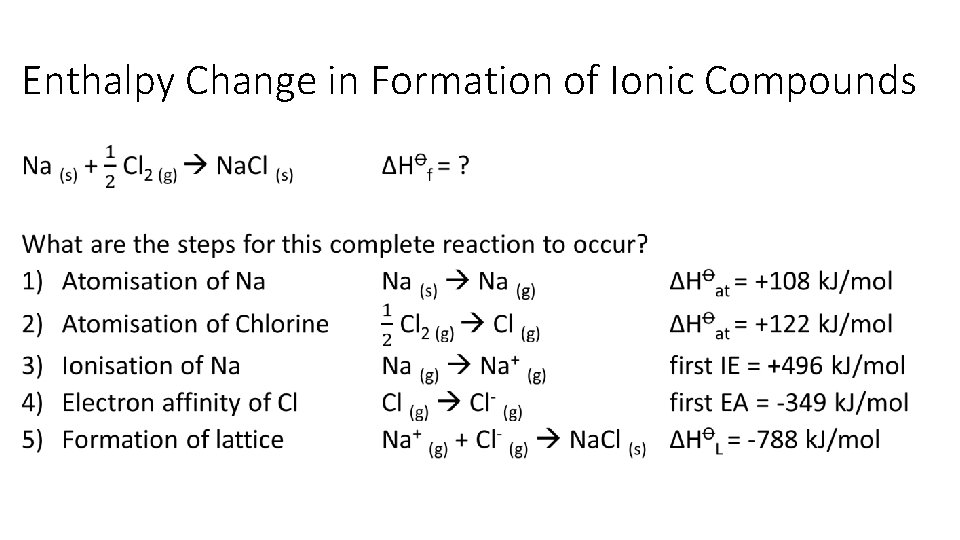
12 Thermodynamics 12 1 Types Of Enthalpy Change

Caie 9701 11 O N 20 Q 6 Enthalpy Changes Youtube

For Part I How Do I Write The Equation For Atomisation I Remember In Enthalpy Change Of Atomisation One Mole Of Atom Is Formed From Substance That Is In Its Standard State

A Level Bond Enthalpy Bond Dissociation Energy Calculations For Enthalpy Of Reaction Ks5 Gce Chemistry Revision Notes
0 Response to "Enthalpy Change of Atomisation"
Post a Comment